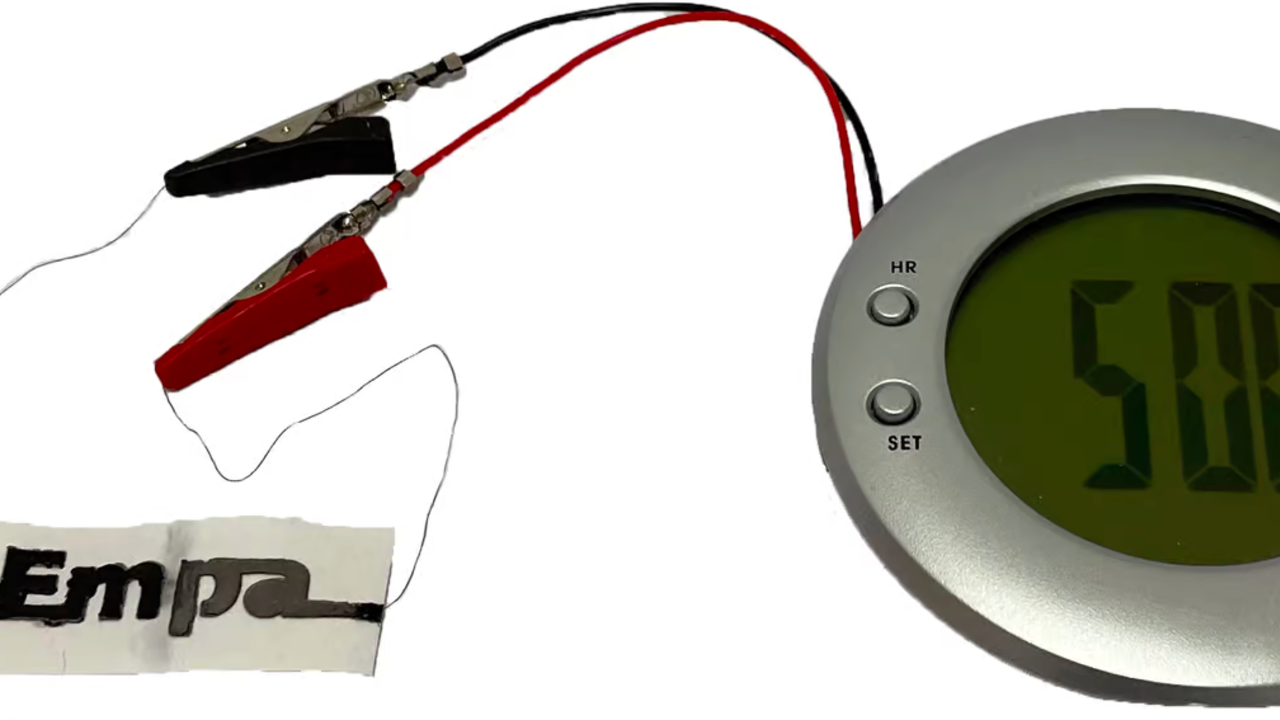
Developed by Swiss scientists, the battery is designed for use in smart transport tags, environmental sensors and disposable medical diagnostic devices.
The battery prototype consists of connected cells. Each cell is one square centimeter in size and the paper substrate is impregnated with sodium chloride (aka table salt). One end of the battery has a wax coating to which two wires are attached. On one side of the paper, ink is applied with flakes of graphite that act as cathodes. On the other hand, there is the ink containing zinc dust which acts as the anode. The positive and negative electrodes are connected by wires to the paint with graphite flakes and soot.
When a small amount of water hits the battery, the liquid dissolves the salt in the paper, releasing charged ions. They disperse in the electrolyte, initiate the oxidation of zinc at the anode and trigger an energy-producing reaction.
Source: Ferra