You have probably seen the didactic diagram of an atom with a nucleus, in which positive and neutral particles called protons and neutrons orbit (just as the Sun orbits the Earth and other planets in the solar system). ) by negative particles we call electrons. However, this is only a representation and does not fully explain how the atom actually is.
The biggest problem here is that it is impossible for the human eye to “see” the atom. Our eyes can only “see” one thing: photons. Photons are subatomic particles found in light, and our eyes can capture photons that vibrate within a certain frequency range. After our brain captures these particles, it interprets these vibrations as colors and shapes in the images we see. In other words, every image we see is the result of our brain’s interpretation of the effect of photons on our retina.
Therefore, if our eyes can detect a single particle, it is physically and biologically impossible for our eyes to actually “see” an atom made up of particles other than a photon! Obviously, when we look at another object, such as a table, a wall, or another person (not made up of photons), what is actually seen are photons that are reflected and reach the surface of those objects. Our eyes. Very small atoms, even with the best microscope, cannot reflect visible light and therefore cannot be seen.
The question that remains is: If we can’t actually see an atom, how do we know that our representations are minimally correct? First, we can understand that our senses, such as sight, are extremely limited and often mislead us when it comes to understanding most natural phenomena. Yet there are many other properties that define atoms and their particles, such as mass and electric charge, for which our eyes will no longer be relevant.
Also, atoms may be too small to reflect the photons required for our vision, but not too small to reflect electrons. So, if we focus on atoms and molecules instead of a beam of light, a beam of electrons, and somehow manage to capture the reflected electrons, we can get a good idea of the size and shape of the atoms. .
This is how electronic microscopes work, one of the best ways we’ve ever had to “see” atomic structures. This type of microscope produces images by capturing the reflected electrons. By knowing the direction in which the electrons strike and where they go after interacting with the atoms, we can have a good idea of the shape and arrangement of atoms and molecules.
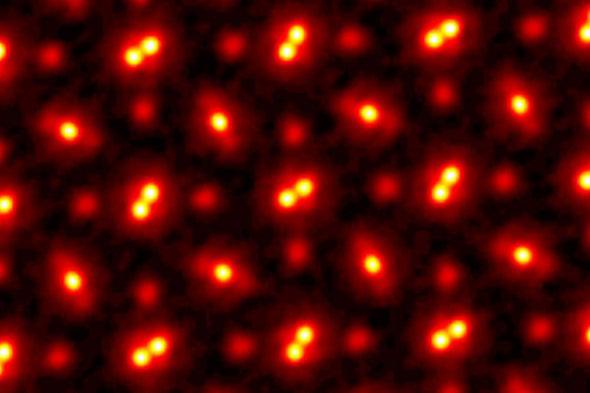
Of course, the questioning can always continue and expand to electrons and subatomic structures. How do we create an image that manages to capture something so small and in constant motion? Again, electrons are particles rooted in physics. It is possible to measure its mass and electric charge with good precision. But in order to have an image, we need to define its position and velocity, which goes against Heisenberg’s uncertainty principle.
This principle states that it is not possible to simultaneously measure the position and velocity of particles with high precision. This does not mean that measurements made in the laboratory are not accurate. The idea is that the harder we try to focus the measurements on an electron’s position, the less we will know about its velocity and vice versa. It’s as if the universe doesn’t want to let us “see” its most fundamental particles. Yet we humans are persistent, capable of analyzing properties and controlling particles that are not even visible. It’s interesting to see that there is beauty in that.
Source: Tec Mundo
I’m Blaine Morgan, an experienced journalist and writer with over 8 years of experience in the tech industry. My expertise lies in writing about technology news and trends, covering everything from cutting-edge gadgets to emerging software developments. I’ve written for several leading publications including Gadget Onus where I am an author.













