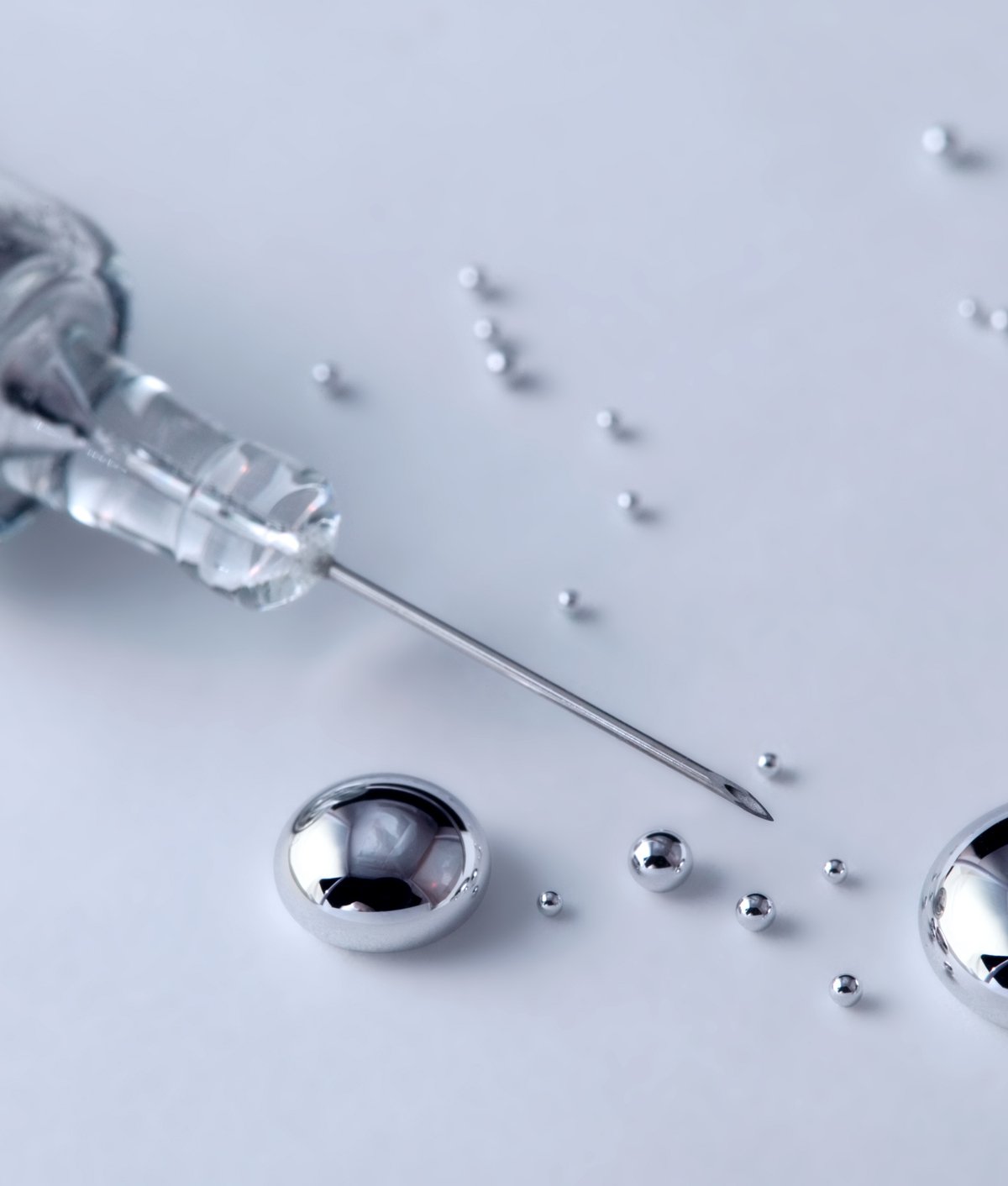
Most solid metals such as iron, aluminum, copper and lead, They can reach melting points of up to thousands of degrees Celsius; from then on they are considered liquid. For example, tungsten needs to reach a very high temperature of 3422°C to turn into liquid. However, there are also metals on Earth that can melt in much colder climates.
Some materials with relatively low melting points turn into liquids at temperatures that would be considered freezing even for humans and animals. In the case of cesium, the melting point is reached only at 28.4 °C. But, There is only one metal that is completely liquid at room temperature; melting point is -38 degrees Celsius: mercury.
The simplest answer The ability of mercury to exist in both solid and liquid forms will depend on the climate in which the material is found.. In any natural region around Brazil, for example, it will always be in liquid form. But if it were in Russia, during the intense winter months the metal would turn into a solid depending on the temperature, but there are cities where temperatures exceed -42 degrees Celsius.
According to Craig Carter, professor of materials science and engineering at the Massachusetts Institute of Technology (MIT), the specific melting point of each metal is related to the bonds of the atoms that make up these materials.
He uses the baseball to explain what’s inside these metals. For example, if a pitcher hits a ball, the ball is thrown in one direction and speed; Again, If there are several microscopic balls, they will move in different directions but with a single speed. This happens due to kinetic energy.
“A metal is full of bonds between the atoms that make it up. What determines the melting point of a material is all about the energy associated with the bonds. Bond formation converts some of the kinetic energy into binding energy. “The bond energy in mercury is very low and tends to become disordered at low temperatures,” Carter said in an official MIT publication.
Mercury is liquid and solid
Some of a metal’s kinetic energy is converted into bond energy, but in mercury the bond energy is so low that not much movement between atoms is required. That is, when placed in an environment below -38 degrees, the distance between the atoms of the material decreases, thus the movement stops and the mercury becomes solid.
Just as water passes through liquid, solid and gaseous states, the metal will become liquid when it reaches higher temperaturesAs atoms become more disordered, this disorder is favored, causing metals to become liquid. In cold climates, atoms are in a solid state because they are more organized.

“Liquids are more disordered than solids, so solids are preferred at low temperatures. If you want to manipulate materials, you need to know how metals behave at various temperatures. Before the advent of composite resin fillings, dentists were filling cavities with a substance that had a very low melting point,” adds Carter.
Melting point It is not the same for all metals found on Earth; If you mix different metals and create a molten alloy, the melting point will also change and the behavior of the material will also change. Later, Although mercury is liquid at -38 degrees Celsius, it can remain solid at normal temperatures when mixed with silver, tin or copper.
In addition to mercury, scientists note that bromine also has good melting point resistance; It melts at -7.2 °C; However, bromine is a non-metallic element from the halogen group.
Two other transuranic elements called Copernicium and Flerovium may also be liquid at room temperature on Earth. Neither occurs naturally on the planet, but both can be produced artificially.
Did you like the content? So, stay up to date with more scientific curiosities at TecMundo. If you wish, take the opportunity to discover how the periodic table emerged.
Source: Tec Mundo
I’m Blaine Morgan, an experienced journalist and writer with over 8 years of experience in the tech industry. My expertise lies in writing about technology news and trends, covering everything from cutting-edge gadgets to emerging software developments. I’ve written for several leading publications including Gadget Onus where I am an author.