A team of chemists from the University of California, Los Angeles, USA, decided to challenge a century-old rule in organic chemistry textbooks: the so-called Bredt rule. This “law” proposed by the German chemist states that double bonds in organic molecules are impossible or almost impossible.
The problem with this principle is that it is an axiom, meaning that it is accepted only on the basis of practical observations, not pure theory, the authors of the study recently published in the journal Science say in a press release.
The new article not only calls for rewriting textbooks, but also How to produce molecules that clearly violate Bredt’s rule, It paves the way for their production and use in reactions. These molecules, purposefully called antibred olefins (ABO), may be “useful in pharmaceutical research,” the statement said.
What is Bredt’s rule?
Bredt’s rule was first proposed in 1902, but was only codified and applied to small organic molecules in 1924. that is, it is carbon-based, in which the atoms are structured into a pair of bonded rings (bicyclics). In this case, each ring is connected to the other by three atoms.
But Bredt notes that it is impossible for these molecules to have a carbon-carbon double bond at any of the “bridgehead” atoms, that is, atoms that act as connecting points between different parts of a structure.
These molecules with “impossible” double bonds are called olefins, and the problem Bredt points out is that carbon-carbon double bonds require atoms to share their electrons. However, the orbits of the bridgehead atoms (where the electrons are located) do not easily approach each other.
How does the study suggest changing the Bredt rule?
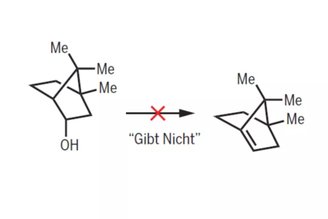
To definitively invalidate Bredt’s rule, the authors show how it is possible to create various types of ABOs that deviate from the traditional olefin geometry taught in current organic chemistry courses. Such an observation for co-author Kenneth N. Trueblood should serve only as a guide and not as a ruleBecause “it destroys creativity,” he concludes.
The recipe for an original ABO involves taking similar silyl halide molecules and treating them with a fluorine source, removing certain elements from these molecules, resulting in the formation of a double bond in an unusual position.
According to senior researcher Professor Neil Garg, “What this study shows is that, contrary to a hundred years of conventional wisdom, chemists can make and use antiredt olefins to make value-added products.”
Did you like the content? Stay up to date with more studies like this at TecMundo and take the opportunity to find out whether we’ve been misinterpreting Isaac Newton’s 1st Law for 300 years. Until later!
Source: Tec Mundo
I’m Blaine Morgan, an experienced journalist and writer with over 8 years of experience in the tech industry. My expertise lies in writing about technology news and trends, covering everything from cutting-edge gadgets to emerging software developments. I’ve written for several leading publications including Gadget Onus where I am an author.












