Alkali metals around group 1A of the periodic table, Almost invisible and undetectable lies an element so rare: Francium. The discovery story is a race triggered by a (vague) certainty about an element that is assumed to occupy the 87th place in Mendeleev’s classification.
Since it was known that the elements react to a certain natural order, it was expected that the gaps in the periodic table that could not yet be filled in the classification of elements proposed by Dmitri Mendeleev in 1869 would soon be filled.
However, it turned out that completing the album of elements in the universe was a much more difficult task than expected. For example, some challenges are linked to the amount of materials available and the lifespan of these elements. Come and learn about Frâncio’s breed and find out if it has any practical uses in daily life.
A Frenchman here, a Frenchman there, but nowhere
The suggestion of element 87 was proposed by Mendeleev in the mid-1870s. In the scientific world, where discovery opportunities are heating up, the search for this element by then began hypothetically.
But the research did not progress as quickly as desired, or at least expected. The first “signs” that Frâncio’s discovery might be possible date back to 1914However, due to the First World War scenario, the experiments were not continued.
Records of the search for the element came back in 1925 with the Russian chemist DK Dobroserdov. Relying on his observations showing the presence of traces of radiation emitted by potassium samples, he even proposed the name “rusium” in honor of his homeland. But this was a red herring.

The same situation happened to other scientists such as Gerald Druce and Frederick Loring (1926).alkaliniumwith “Fred Allison (1930),”virginity” and with Horia Hulubei and Yvette Cauchois (1936), “Moldavium”.
Anyone who had the courage to publish their discoveries was subjected to great criticism of their work, as the methods of observing, detecting and managing resources appeared inadequate.
Let’s say the news was slower but no less controversial. But in 1939, a wave of radiation caught the attention of Marguerite Perey. When examining the actinium example, we examine element number 89 in the periodic table. He observed the element’s unusual radiation emissions and chose to investigate this “invisible” source.
At the time, Perey was working in Marie Curie’s Laboratory and became the first person to study the properties of the element, which had recently been isolated from a natural sample of Actinium. Eventually, Frâncio was discovered, named in honor of its discoverer’s birthplace..
Rare and temporary
It is still unclear what Frâncio looked like since his discovery, because it has never been possible to obtain a sample large enough to actually observe it.
It occurs naturally through the decay of elements from the uranium family, so it is a radioactive element. It is so rare in the Earth’s crust that it is believed that no more than 30 grams of the element is present at any given time.Even if they unite every tiny atom spread throughout the world.
Its half-life is also quite short; for the most stable isotopes it is about 22 minutes. Of course, there are elements with a much more temporary existence, lasting milliseconds, but these are usually of synthetic origin. Francium is considered the last discovered element that can be isolated naturally..
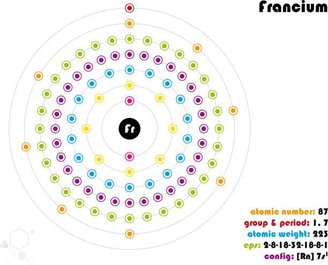
Due to its rarity, abundance, difficulty in obtaining and possible reactivity, it has no applicable use in daily life as it is limited to research laboratories.
It can also be “produced” synthetically by bombarding elements such as Radium and Thorium. Bombardment serves to “tear up” particles such as electrons that reach the desired number to obtain new elements. To achieve this through Radio, the technique of bombarding the atomic nucleus with neutrons is used. Thorium needs to be bombarded with protons.
Although right now Although element 87 has no everyday applicability, this work helps us understand the fundamental behavior of atoms and gives us clues about other possible fundamental forms in the universe..
Until enough Francium can be produced for further study, here’s a look at how quantum study could explain the mystery about radioactive nuclei that are as unstable as cotton candy in the rain. Until next time!
Source: Tec Mundo
I’m Blaine Morgan, an experienced journalist and writer with over 8 years of experience in the tech industry. My expertise lies in writing about technology news and trends, covering everything from cutting-edge gadgets to emerging software developments. I’ve written for several leading publications including Gadget Onus where I am an author.