Chemistry depends on the ties and forgiveness of the components to produce the world and everything we know. These chemical connections are not just desired, However, it is necessary to have atomic stability. Everyone wants a little quiet in their presence.
A good part of the periodic table elements reaches this balance by obtaining eight electrons in the final value layers, which is called the octet rule. However, some of them are less demanding and do not need to keep all of these to remain constant, such as helium, berillium, hydrogen, aluminum, lithium, and of course the star of the day: Boron.
Since everything is not purchased on the market or on the internet to buy electrons, the elements must be mutually opened to donate electrons or through sharing.
However, as in the macro, the disaster of the disaster, the deterioration of these connections can create parties and very useful explosions for energy production.
And the new discovery of researchers at the University of Würzburg, Germany, can give us an extra connection to break and produce interesting things. Not because we’re interested in the bomb end.
Connects the world: chemical bonds
Chemical connections The paths of atoms living and the world built. They can occur in different ways with ‘donation’ or electron sharing. The main calls are called ionic and covalent bonds.
In ionic connections, there is a donor atom and a receiver. When this is the electron transfer, the polarity of the atoms may occur, so that the nuclei draws themselves by forming the molecule.
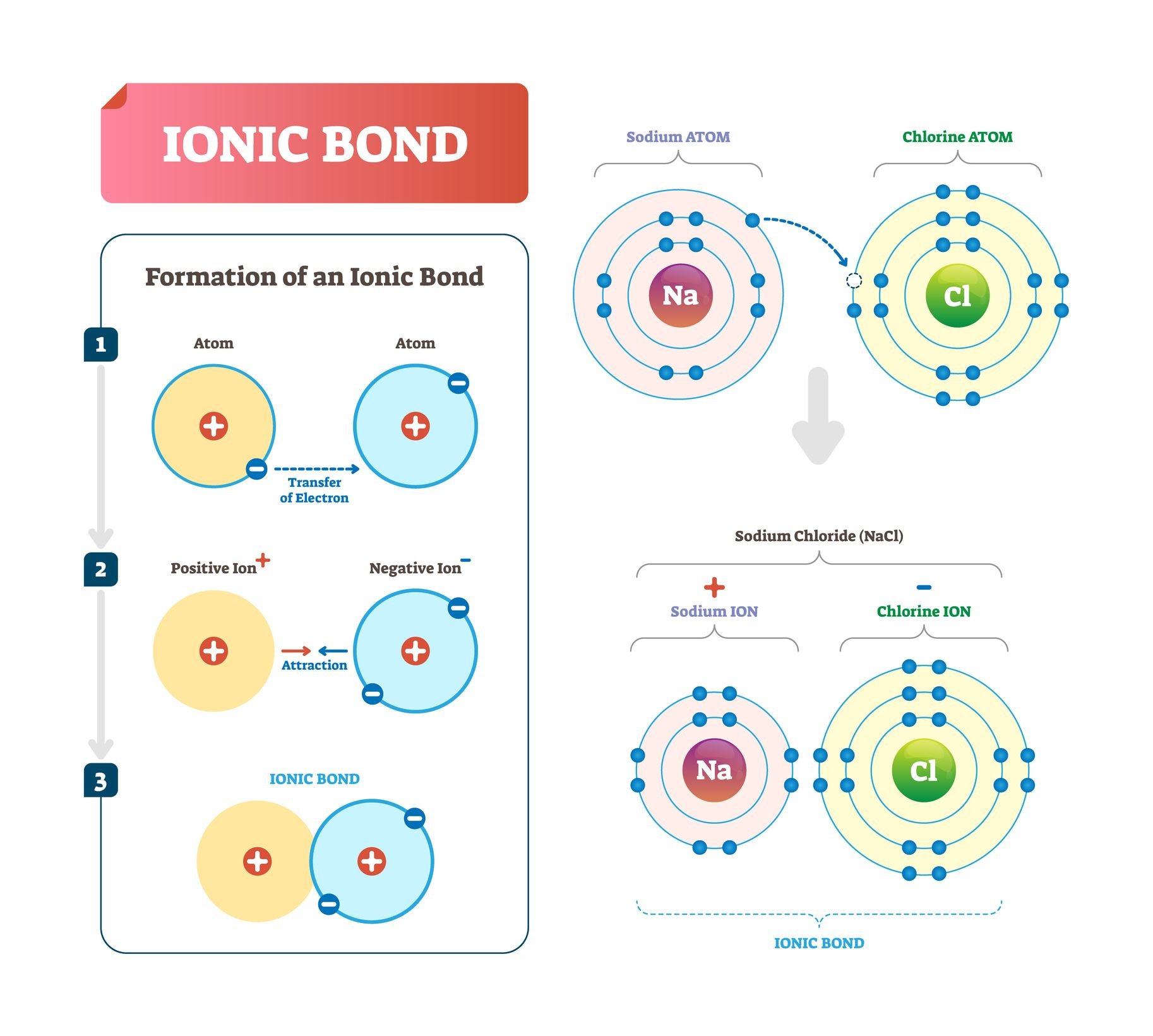
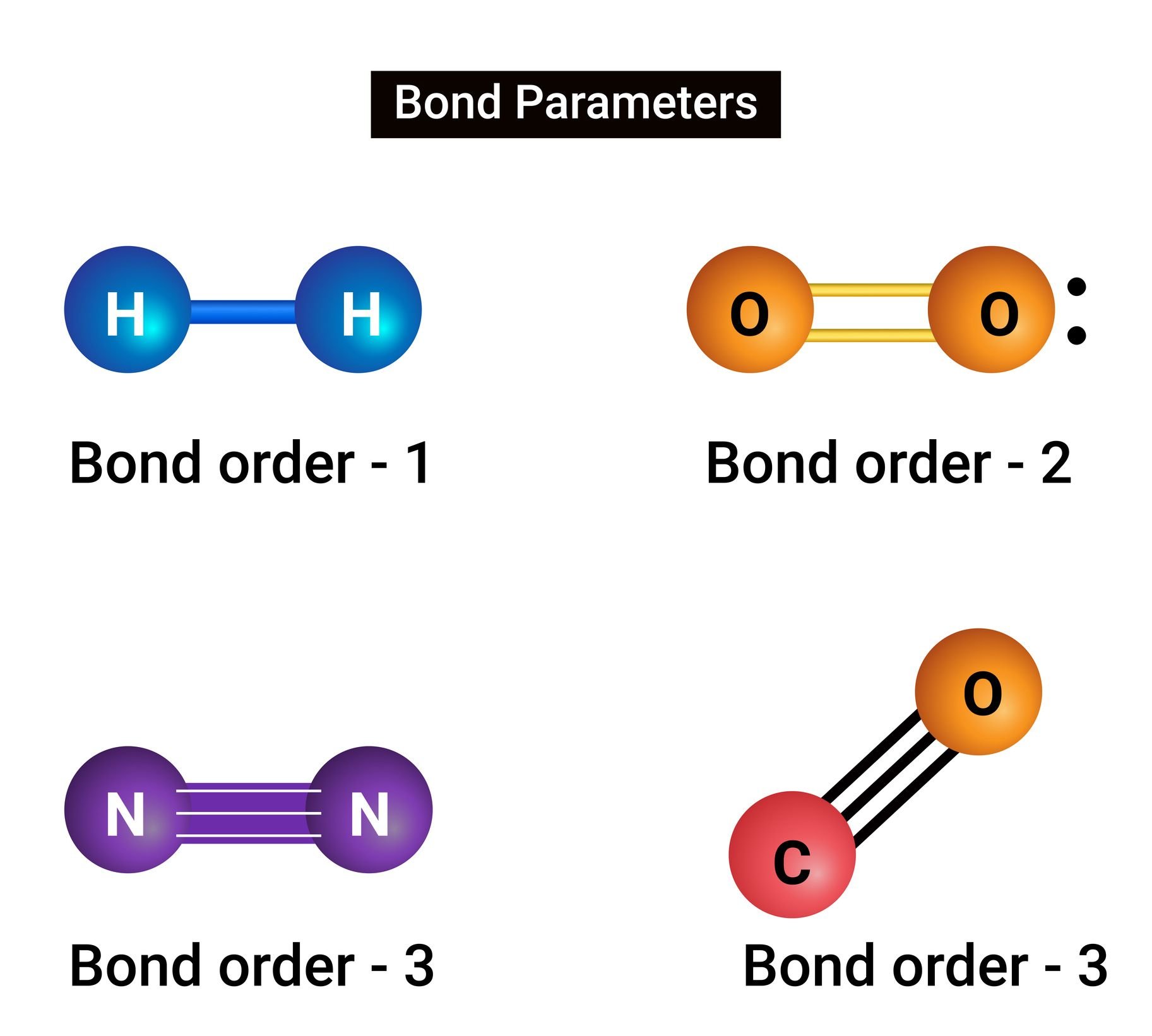
However, if there is no possibility to donate, calls can be produced by electron sharing. In covalent vineyards, multiple dual electrons can be associated, so connections can be simple, double, triple or datif.
This joint use of the electrons of the final value layer will depend on the need to reach the electronic stability of each nucleus, so all elements will not be talented or will not need to produce multiple connections. However, there is often a group of triple bonds with each other.
Oxygen, nitrogen, boron and carbon are known to produce triple -related molecules. However, even if they have more sensitivity, As in the case of boron and carbon, some of these arrangements have not yet been observed.. But it was the past.
Bor-Carbon or Borino for sincere
For the first time, researchers at the Julius-Maximilians in Bavaria, Germany, produced a molecule with a triple connection between carbon and boron at the University of Würzburg. Molecules with double bonds were already known and not rare. The research was published in Nature Synthesis.
To produce feat, components arranged in triple -linked molecules when exposed to boron and carbon atoms and controlled pressure and temperature in group ion traps. According to the study group, some thoughts can be made from this magnificent result.
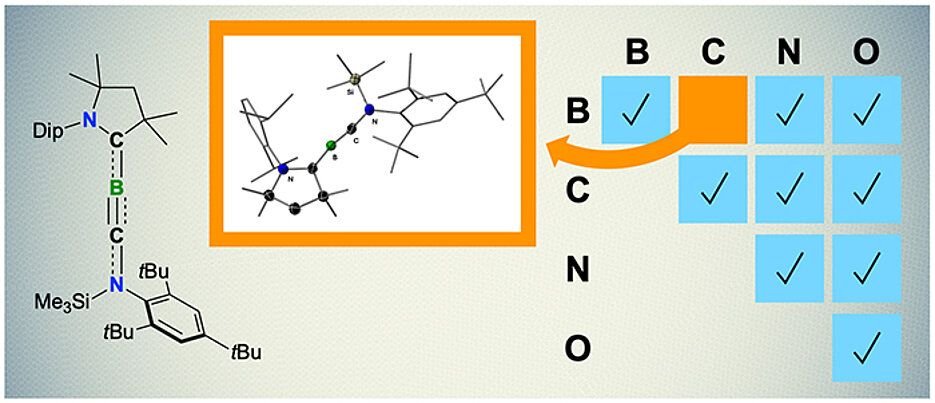
For example, even if it is possible to form the molecule called Borino at that time, it has been observed to be “disturbing ında in a linear connection with boron carbon. Some atoms have binding ideas, even if some are more “flexible .. Connections produced at very extreme angles can be fragile. This provides reactivity to these molecules.
In a banal example, the table you insist on adjusting without instructions and without the right tools. He’s standing, but if someone gets close, he’s completely dismantling.

However, the “easy -to -break” things can be very useful, so new research steps will be aimed at understanding how this triple connection can remain stable and the effects of the refraction reaction of these molecules.
In molecular tetris, angles and reactions are necessary for the development of new drugs and the development of useful molecules for energy production and energy storage.
Naturally, this type of boron and carbon connection may not be detected before due to the need for pressure and temperature control to keep the boron position constant. Who would say that periodic painting elements could also be back pain?
The research group reflects the importance of “apparently” banal research by showing the discoveries that contribute in different ways in daily life, such as the development of Teflon and Supercola.

The advancement of technology is closely linked to the observations we make in the laboratory or in the mixture of the right materials in daily life. It contributes to knowing how the elements work, connecting and how their chemical connections work, to generalize their use or to get new functions.
Ozpic, for example, is a drug that was initially produced for the control of diabetes, but the mechanism of action of the molecules in the drug also induces the physiological effects that contribute to the treatment of weight loss.
Therefore, we still don’t know what the potential of the triple molecules between boron and carbon are, but this unprecedented venture can bring interesting results to the development of future new technologies.
While waiting for news, learn a little more about the periodic table starting from the element that constitutes 78% of the atmosphere: nitrogen! Keep tracking Tecmundo for more news and tell me what you want to know about science, life and the universe in our networks! Until later.
Source: Tec Mundo
I’m Blaine Morgan, an experienced journalist and writer with over 8 years of experience in the tech industry. My expertise lies in writing about technology news and trends, covering everything from cutting-edge gadgets to emerging software developments. I’ve written for several leading publications including Gadget Onus where I am an author.