Aging is an irreversible path. Since fertilization, our cells begin a natural aging process that will contribute to cellular maturation, enable our vital units to reach maximum functionality, and also signal to the organism that it is time to say goodbye.
‘Old’ cells or cells that have been highly damaged by internal or external factors such as irradiation, trauma or even pathologies, It activates the self-destruction process called cellular apoptosis, which is responsible for carrying out this recycling.
However, some cells can escape this fatal outcome by turning into a kind of zombie. In a new study published in the Journal of Aging Cells, researchers from the Mayo Clinic discovered that senescent cells that accumulate in the skin drive very atypical functioning by stimulating faster aging of other cells, systems, and structures in the brain.
To understand A little more about zombie cells Check out the interview with skin dwellers and study co-author and researcher Nathan LeBrasseur, Ph.D.
Why “zombie cells”?
It may seem strange to say this Skin cells have the ability to stimulate brain structures to age. However, we should not forget that our body is a one-piece machine and each cell can produce signals to its environment.
In the case of the epithelial cells described in the study, they underwent an aging process that, over time, evaded normal cell death mechanisms such as apoptosis, which would cause the already damaged cell to die.
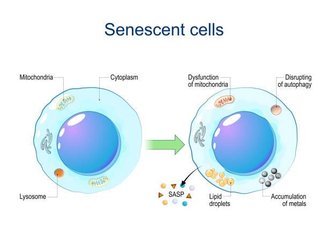
The cell enters a dormant state by stopping its reproductive processes as its own defense method to avoid producing defective copies of itself. But it is not active. It continues to emit chemical signals in paracrine type signaling.
Upon receiving these signals, neighboring cells are stimulated to enter senescence, triggering an aging process in the neighborhood. And this communication can extend to organs, including the brain.
Because of this dichotomy between a dormant yet chemically active state, researchers have called these senescent epithelial cells “zombies.”
mobile phone thriller
To test their hypothesis, the research team performed cell transplants into mice. They induced donor epithelial cells to undergo senescence.
Following this process, they identified these cells and stained them with specific biomarkers that would help them confirm the signaling pathway of the cells after transplantation.
This cell preparation was transplanted into the intradermal layer of young rodents; After months of monitoring, clear signs of tissue aging emerged, such as loss of fat beneath the layer of skin where the zombie cells were located.
Moreover, recipient mice began to show signs of cognitive decline, changes in the structure of the brain’s hippocampus, and muscle and bone loss.
Aging is natural without panic
Although such studies show that aging is an infectious disease, this is not the main purpose of the research.
These findings are extremely important, after all, we transplant organs, not just cells. Understanding how donor cells may interact with recipient cells contributes to the development of new treatments. Aiming to minimize damage and increase the lifespan and viability of transplants.

It also allows us to think about ways to promote healthy aging, not only about drug treatments but also about lifestyle, such as maintaining physical and intellectual activities and the importance of adequate nutrition.
If you want to learn a little more about the research, check out Dr. Check out the email interview with Nathan LeBrasseur.
Learning more about zombie cells
Question: Cellular aging is a natural process that can be stimulated by agents outside the organism and often leads to cell death. In this context, what causes “zombie cells” to remain in tissues rather than being destroyed by the body?
Reply: Cellular aging is a natural and often beneficial process that occurs throughout life. For example, cells undergo senescence as part of embryonic development and as a primary defense against the growth and spread of cancers.
Our body’s cells go through various forms of internal wear and tear, which can be affected or accelerated by external stressors (such as chemotherapy, UV radiation, or even excess nutrients). The resulting accumulation of different forms of molecular and cellular damage reflects biological aging.
While cells can repair some damage and continue functioning, others become so damaged that they die; This process is known as apoptosis. Senescence is a cellular fate in which cells persist despite damage; hence the reference to “zombies”..
They activate mechanisms that halt cell division, prevent death, and produce a wide variety of cytokines, chemokines, growth factors, remodeling proteins, and other bioactive molecules (collectively known as SASP or senescence-associated secretory phenotype). These cells behave differently than healthy cells.
In youth, senescent cells appear but are effectively identified through SASP and destroyed by the immune system. As we age, increased wear and tear causes more damage and more senescent cells. while the immune system becomes less efficient at identifying and eliminating them.
So they accumulate. We understand that aging is initially beneficial, but if these cells persist, they promote aging-related disorders, dysfunctions, and diseases, largely dependent on SASP.

Question: Is it fair to say that zombie cells have the ability to infect healthy cells, rendering them inactive or more prone to inactivity?
Reply: The intact secretome of not “infected” but senescent cells can strongly influence the behavior of neighboring and even distant cells. They can pollute the environment, It compromises the ability of stem cells to function, stimulates fibrosis and inflammation, and ironically even accelerates the growth of malignant cells.. SASP can also induce senescence in other cells, reinforcing references to “zombies” (not by bites, but…).
Question: Does the formation of “zombie cells” begin in the skin and spread to other organs, or could this simply be a sign of a systemic process?
Reply: Interestingly, not only do individuals age at different rates, but a person’s organs also age at different rates. There is great interest in the development of biomarkers or biomarkers of aging.
But we do not currently know whether markers in the blood or skin reflect the burden or activity of senescent cells in the brain, heart, lungs, liver, kidneys, or other organs. We are going through an exciting time in science and we expect significant advances in this field in the coming years.

Question: Are there any strategies that can help prevent the pathological accumulation of inactive cells in the skin?
Reply: Aging is universal, progressive, and inherent as explained above. But it is also malleable. Healthy lifestyle factors such as physical activity, balanced diet and sleep can positively impact our defenses and ability to repair various age-related damages. Reducing the accumulation of senescent cells in organs throughout the body, including the skin.
Since DNA damage and inflammation are major drivers of aging, strategies to protect the skin against UV radiation, cigarette smoke, and air pollution may help prevent the accumulation of senescent cells.
Question: Is there anything else to add?
Reply: There has been great interest when researchers at the Mayo Clinic and other institutions have shown that senescent cells not only contribute to aging, but that eliminating them in experimental models can positively affect the health and function of many organs.
Strategies to eliminate senescent cells (“senolytics”) or suppress SASP (“senomorphic”) have the potential to delay senescence. prevent or treat a wide range of otherwise age-related diseases and prolong your health. Although more research is needed, these approaches have the potential to transform human health.

Did you enjoy learning a little more about aging and how our bodies can produce their own zombies? Then you might also be interested in knowing the story of the immortal Henrietta Lacks.
Stay tuned to TecMundo and let us know what you’d like to see here on our social networks. Until later!
Source: Tec Mundo
I’m Blaine Morgan, an experienced journalist and writer with over 8 years of experience in the tech industry. My expertise lies in writing about technology news and trends, covering everything from cutting-edge gadgets to emerging software developments. I’ve written for several leading publications including Gadget Onus where I am an author.