On the night of July 4, 1054, countless people across the Asian continent saw a bright and intense light in the sky that could be seen for several days. Chinese astronomers recorded this event in the constellation Taurus as a “guest star”..
That same night, other people on the other side of the globe recorded the same phenomenon: the Anasazi, a culture rich in astronomical tradition, in the southwest of what is now the United States, also witnessed this bright new star. Easily visible in broad daylight, observers can read and admire at night.
We now know that both the Chinese and Anasazi witnessed a stellar explosion called a supernova, and that in addition to light, the dying star released chemical elements into space, giving rise to enormous amounts of energy in the process.
Inside the star that gave rise to the Crab Supernova (SN 1054), there were more than the first 26 elements of the periodic table, from simple elements like helium and carbon to more complex elements like manganese and iron. Other elements were also created during the explosion and pulverized into interstellar space. They eventually came together to form the ions and molecules that form new stars, planets, and large nebulae.
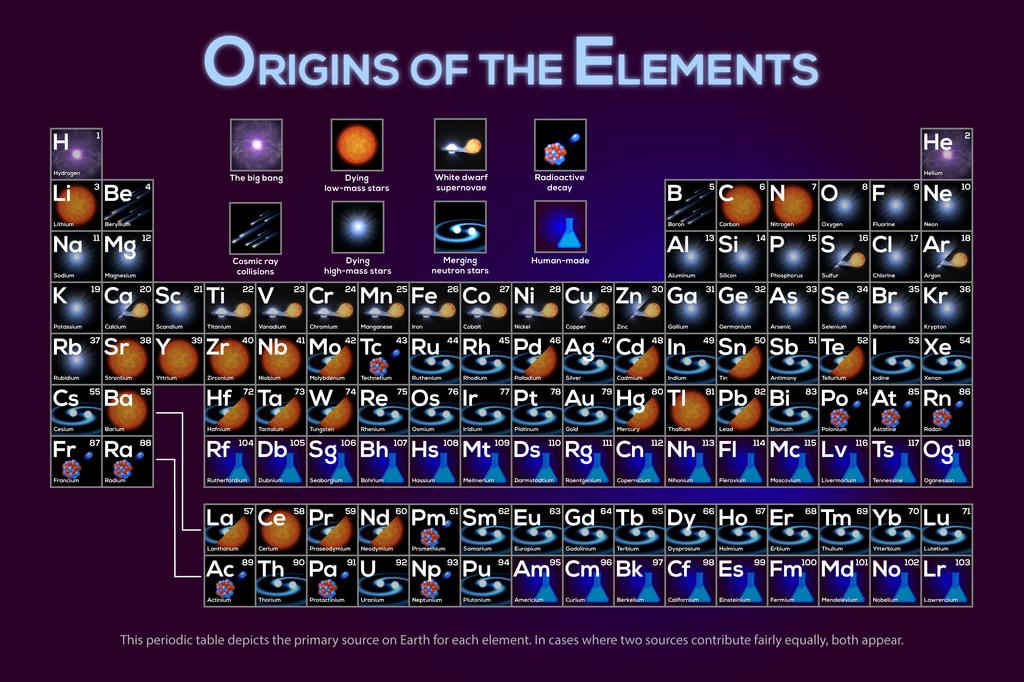
This chapter summarizes much of the history of the heavy chemical elements in the Universe: they were all forged either in the cores of stars or during the dramatic ends of their lives. Today, astronomers recognize these chemical elements scattered throughout the cosmos. It is possible to know the amount of oxygen in the atmosphere of a distant planet, the percentage of iron in a distant molecular cloud, and even whether a particular place has a water molecule.
How is this possible when you have never left the Solar System?
The answer to that question is the developmental story of one of the most incredible techniques mankind has ever discovered: spectroscopy. Although some properties of light have been known since the ancient world, it was only in the 17th century that the development of advanced analysis techniques took place in the Western world, with the publication of the first works on optics by the physicists and mathematicians Isaac Newton and Christian Huygens.

Over time, the development of the first optical devices, particularly prisms, enabled systematic observations of the solar spectrum: Newton was the first to use the word spectrum to describe the rainbow of colors that come together to form white light. At the beginning of the 19th century, Spectroscopy became a more precise and quantitative scientific technique and has since played an important role in chemistry, physics and astronomy.
In optics, a spectrum is a simple table or graph showing the intensity of light emitted in an energy range. Nature often creates beautiful ghosts: rainbows. Sunlight passing through water droplets in the atmosphere is scattered to show its various colors. This is how our eyes perceive radiation with different energies.
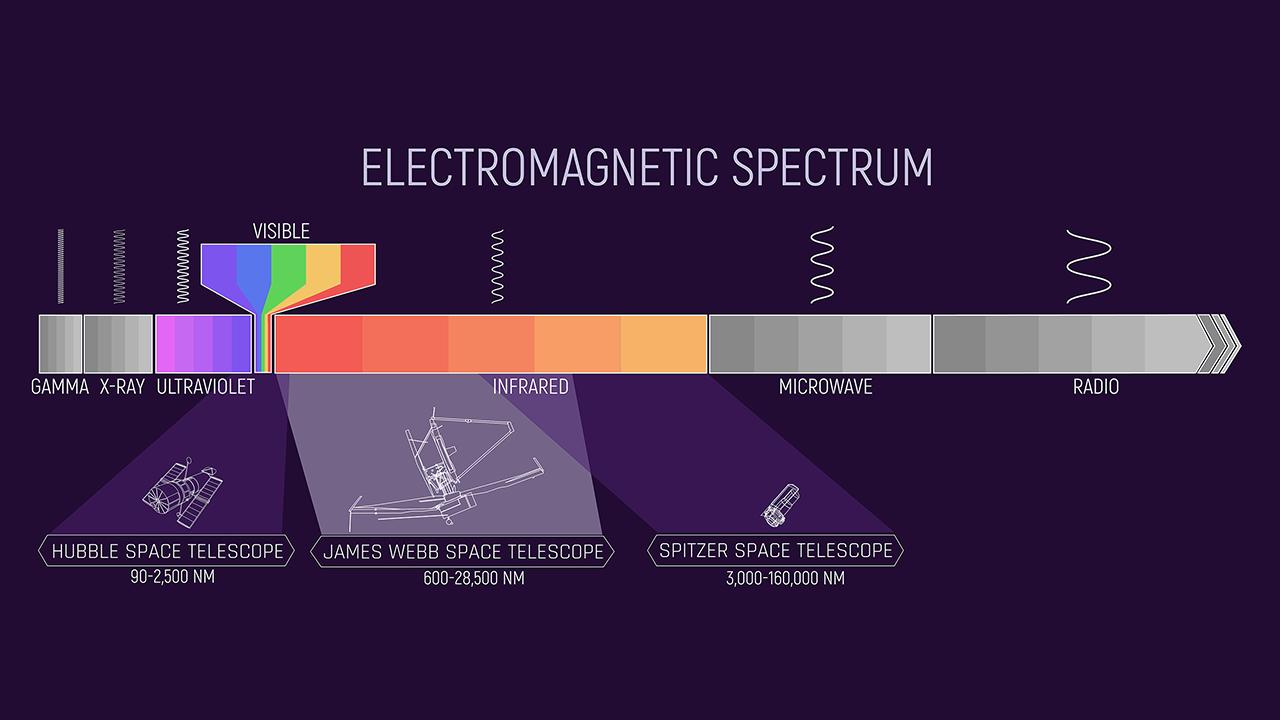
Each spectrum contains a wide variety of information for the simple reason that there are many different mechanisms by which an object such as a star or galaxy can produce light. Each of these mechanisms produces a characteristic spectrum.
Each chemical element in the periodic table produces a series of bright lines unique to that element, like a fingerprint in the light spectrum. For example, hydrogen will have bright streaks, unlike helium, unlike carbon.
Thus, when observing a distant celestial body, astronomers can determine what kind of matter is in it by analyzing the light coming into their instruments. This is the study of spectroscopy, which makes it possible to determine the chemical composition, as well as to predict the temperature and density of these elements (for example, in stars).
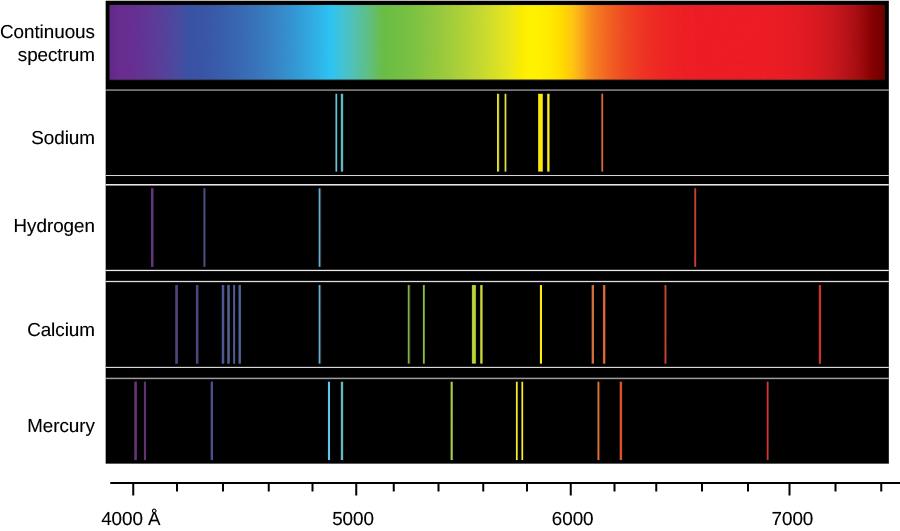
But it doesn’t end here! Spectral lines also give secrets about the magnetic fields of stars, their mass and size, the speed of movement of an object.
Despite being one of the primary tools for studying the universe, spectroscopy has wide application in the most diverse fields of human knowledge, from the study of materials to the most advanced surgical and medical treatment techniques.
Without spectroscopy, we would certainly be groping in the dark, even with light.
Source: Tec Mundo
I’m Blaine Morgan, an experienced journalist and writer with over 8 years of experience in the tech industry. My expertise lies in writing about technology news and trends, covering everything from cutting-edge gadgets to emerging software developments. I’ve written for several leading publications including Gadget Onus where I am an author.