Recently published in the journal Nature Chemistry A new study revolutionizes the way we understand the structure of water at the interface of simple electrolyte solutions. According to the authors, the distribution of ions at the air/water interface is different from what is taught in current textbooks.
In Traditional Chemistry, we learn that larger ions tend to be surfactants, getting their position right above the water surface and triggering electric fields that determine the interfacial structure of water. In the new study, the authors clearly challenge this view by using spectroscopy techniques to obtain more sensitive and selective measurements.
Researchers performed molecular dynamics simulations to evaluate the functioning, speed and efficiency of chemical reactions. The two techniques showed that “the ions in typical electrolyte solutions are actually located in a subsurface region.”
Examination of the water molecule with a more advanced technique
To test their hypothesis, the authors focused on the exact point where air and water meet in the electrolyte solution (salt water) to observe how water molecules behave. This is usually done with a laser radiation technique known as vibration collection frequency generation (VSFG), which measures molecular vibrations.
But because this tool couldn’t measure whether the signals were positive or negative, the team decided to use a more complex model called a heterodyne-sensing VSFG, which can examine different electrolyte solutions. With the help of advanced computational models, the authors then simulated the interfaces in different scenarios.
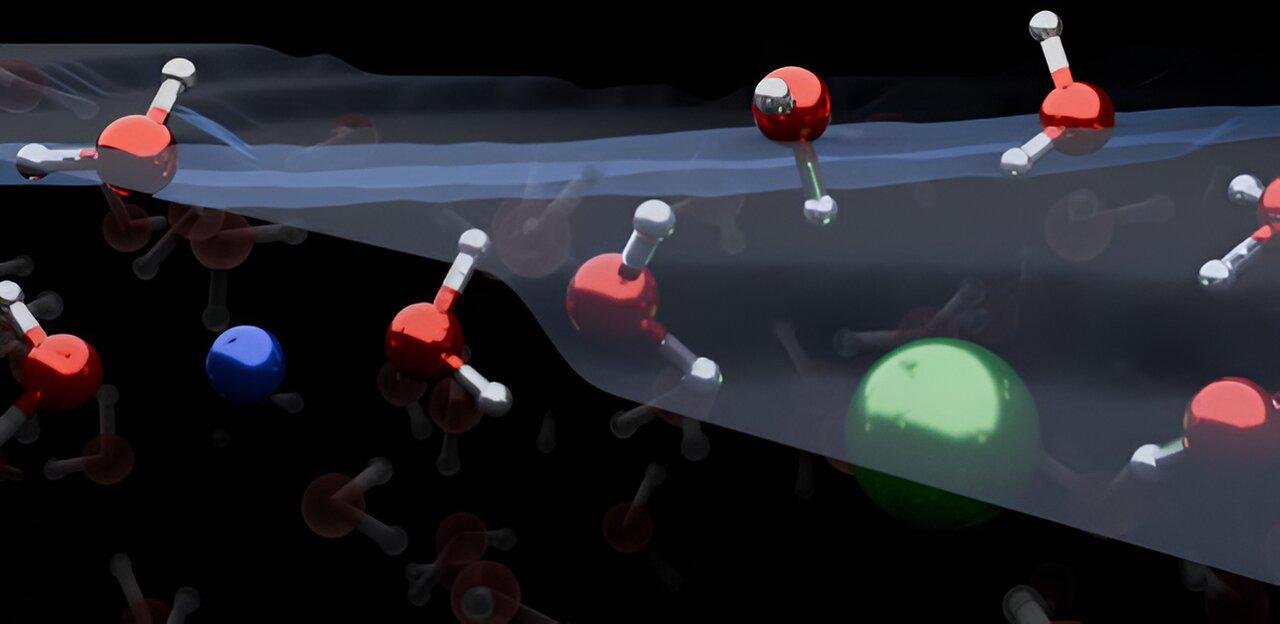
Combining the results, they noticed that both cations (positive ions) and anions (negative ions) were reduced at the water/air interface. This is because the cations and anions of simple electrolytes Turns textbooks upside down by directing water molecules both up and downshowing the formation of an electrical double layer that orients molecules in only one direction.
What are the implications of the new view of water molecules?

The fundamental change brought about by this work was the discovery that the surface of a simple electrolyte solution is divided into layers: a non-ionized outer surface and another ionized sub-surface, which together determine the interfacial structure of water; in this case, this layer refers to the molecular organization of water near dissolved ions.
Lead author Yair Litman, from the University of Cambridge, explains in a statement that “the ion-enriched subsurface determines how the interface is organized.” At the top are several layers of pure water, then a layer rich in ions, and finally the main part, the brine solution.“.
According to co-author Mischa Bonn, a professor at the Max Planck Institute, the new information about water molecules can be used to “study solid/liquid interfaces that may have potential applications in batteries and energy storage.”
Follow the latest scientific research at TecMundo and get to know Nature’s recently discovered new Leia.
Source: Tec Mundo
I’m Blaine Morgan, an experienced journalist and writer with over 8 years of experience in the tech industry. My expertise lies in writing about technology news and trends, covering everything from cutting-edge gadgets to emerging software developments. I’ve written for several leading publications including Gadget Onus where I am an author.