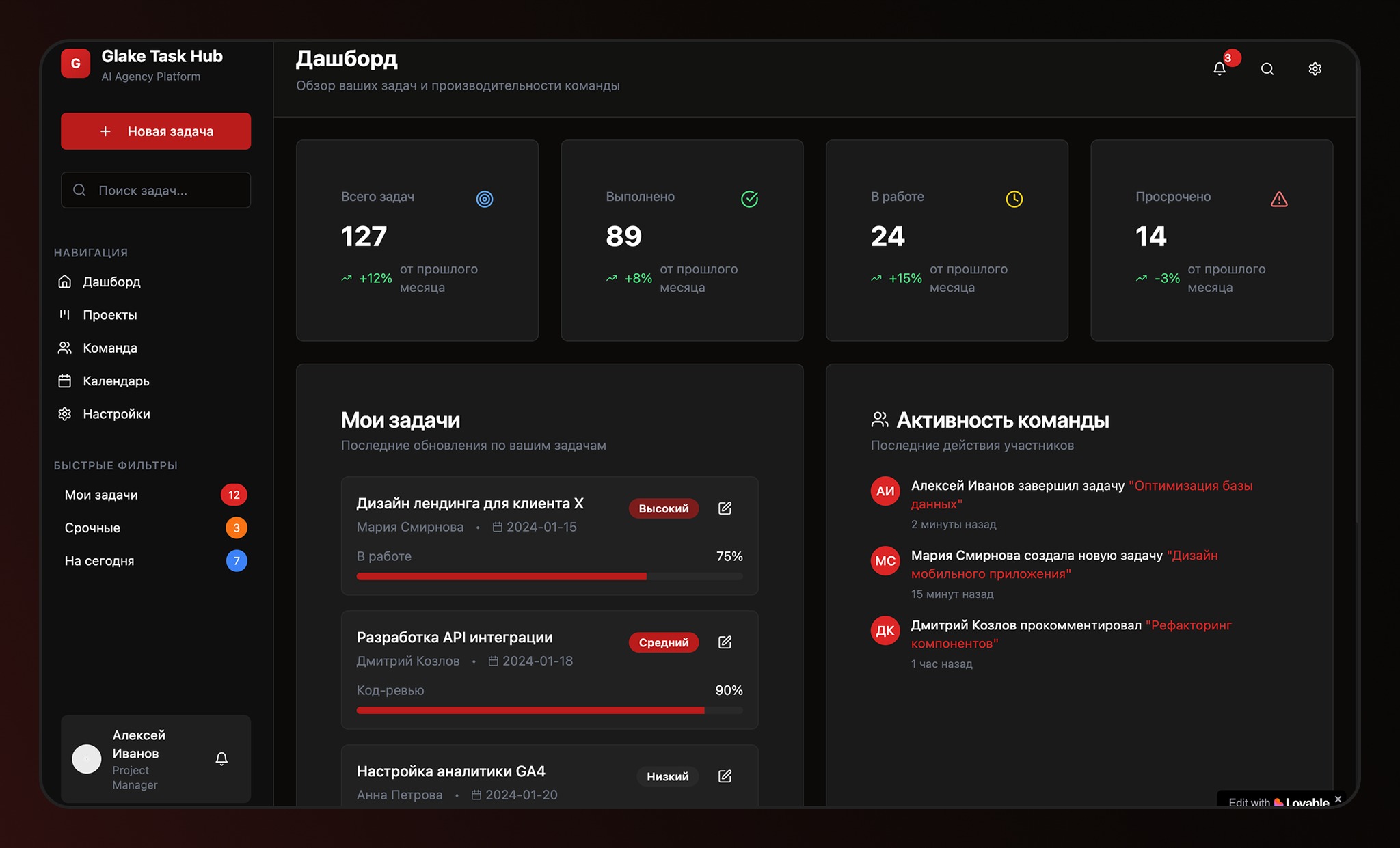
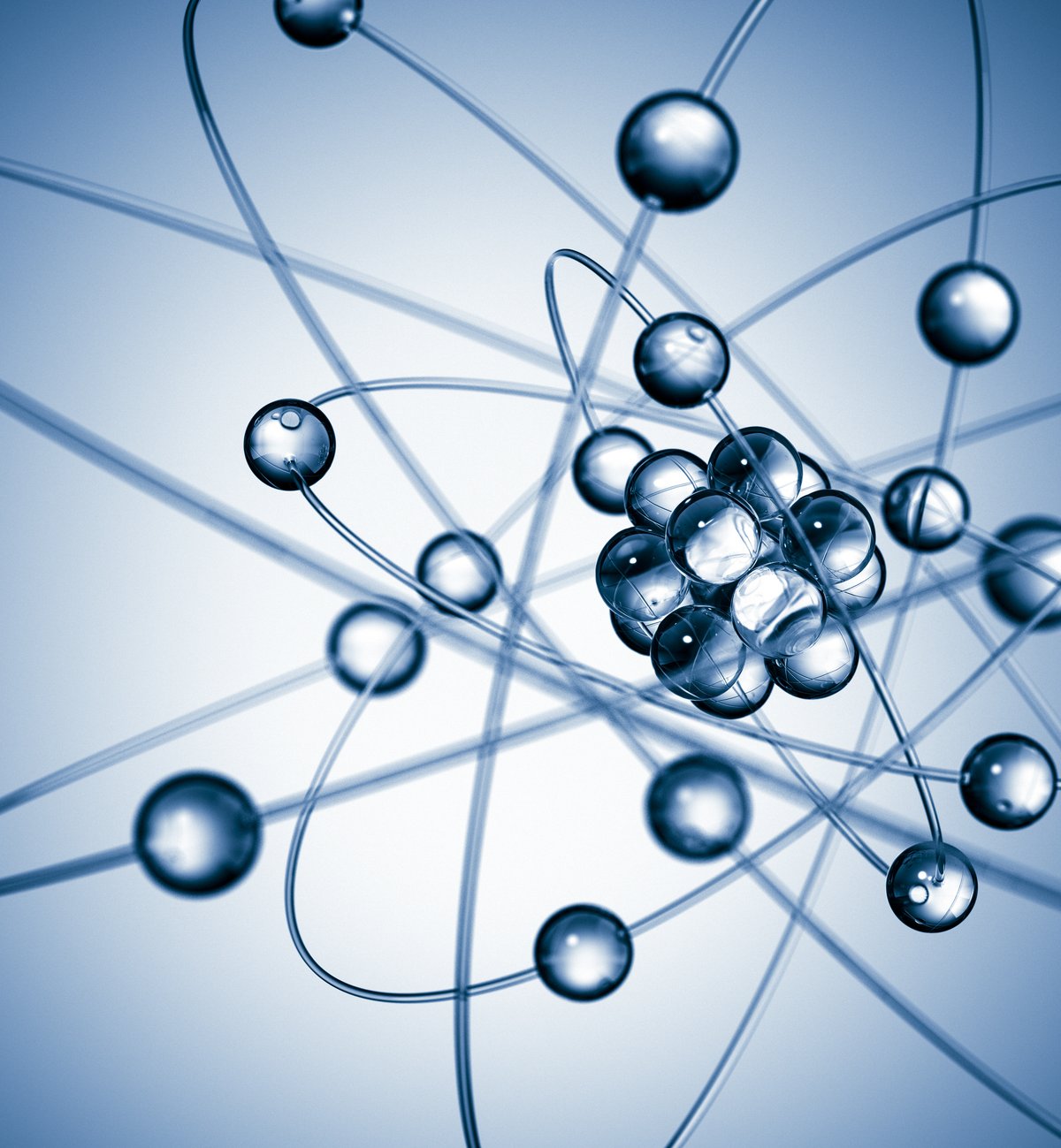
Electrons are fundamental subatomic particles with a negative electric charge and, along with protons and neutrons, are responsible for the formation of atoms.. Even though you cannot observe them with the naked eye, Electrons are essential to all matter around us.R. Many people have heard that a subatomic particle rotates because of its ‘spin’, but that’s not exactly how it works.
In previous studies based on quantum mechanics, many researchers stated that electrons do indeed spin, but not everyone agreed with this hypothesis. Because some observations and Scientific experiments showed the opposite: They said that it was not possible for electrons to spin. – at least not the way we think about spinning.
Scientists explain that there are some reasons to think that an electron can spin, since it is a very small particle and has some specific properties. On the other hand, there are also various explanations as to why an electron cannot actually spin in a common spin as we know it.; in order to provide correct values of magnetic and angular moment, it must rotate at a speed greater than the speed of light.
“Superlight speeds are avoided because the mass and charge of the electron are spread over distances so large that neither the speed of mass flow nor the speed of charge flow need exceed the speed of light. The gyromagnetic ratio of the electron is twice the expected value because its charge is spinning twice as fast as its mass,” explains a published study on the subject.
So can electrons spin or not? In order to explain the subject in more detail, I have collected some information from scientists and other experts in the field.
Are electrons spinning or is it an optical illusion?
Electrons are fundamental particles that make the world we know possible. As they are responsible for many fundamental roles like the formation of atoms and molecules. For a long time, scientists thought that electrons rotated because of their spin, a property associated with the quantum angular momentum of this fundamental particle.
However, spin does not represent a physical rotation of the electron; rather, it represents an intrinsic property of electrons that affects their behavior and interactions..
Most scientists states that the spin of electrons can be understood as a property that simulates the effect of rotation due to their angular momentum, It’s similar to what happens when the Earth spins on its axis. Some physicists even believe that electrons are spinning, but this idea doesn’t imply a physical rotation.
If this were true, such particles would have to be spinning faster than the speed of light, and current physics dictates that it is not possible to go faster than the speed of light. However, it is important to emphasize that This restriction does not apply to the spin of electrons as it is discussed within the concept of quantum mechanics.
The quantum world and the spin of electrons
Early quantum mechanics scientists did several experiments that suggested the spin of electrons, but this idea was a simplification. The current scientific community believes that these fundamental particles do not rotate in any physical way as we know them. Instead, spin is a quantum property that cannot be reproduced in experiments like ordinary physical rotation.
“Philosophers tend to be interested in problems that have long been unsolved. In quantum mechanics, we have ways of predicting the outcomes of experiments that take spin into account, which works very well for electrons, but important fundamental questions remain unanswered: Why do these methods work and what is going on inside an atom?” said Chip Sebens, professor of philosophy at the California Institute of Technology (Caltech).
According to Sebens, electrons are not just particles that appear to be spinning, but are physically spinning “bubbles of charge.” He believes this idea can be explained by quantum field theory, which suggests that fields are more fundamental than particles. Particles and fields are fundamental concepts in physics, but there is still debate over which is more fundamental.
It is important to emphasize that This area remains widely investigated, and there is no definitive confirmation of the concept of electron spin.
Did you like the content? Tell us about it on our social networks and take the opportunity to discover more about the fantastic and strange world of quantum physics. Until next time, Science has the answers!
Source: Tec Mundo
I’m Blaine Morgan, an experienced journalist and writer with over 8 years of experience in the tech industry. My expertise lies in writing about technology news and trends, covering everything from cutting-edge gadgets to emerging software developments. I’ve written for several leading publications including Gadget Onus where I am an author.