The wild card of the universe is hydrogen. The universe is full of it, and almost all carbon-based compounds contain molecules of this element, which ranks first on the periodic table. The sun, the water you drink every day, your body, and most everything you touch are born from a combination of this element..
It can be used in many ways, from the deadliest to the most beneficial. For example, it has emerged as a promise to develop plans to replace fossil fuels and decarbonise industrial processes and transport.
But, The use of hydrogen has already led to more destructive uses, such as hydrogen bombs, which have far surpassed atomic bombs.It is based on plutonium and uranium. Learn more about the history, discovery, and everyday applications of this element.
Hydrogen: How did it all begin?
Even though it is the most abundant element in the universe, it took someone some time to identify it. According to historical data, The first mentions of the isolation of hydrogen date back to the 1500s, to the physician and alchemist Paracelsus..
According to reports, he dissolved iron filings in a solution of sulfuric acid. This mixture produced flammable bubbles (gases). However, he did not continue to investigate the effects of the mixture’s reaction, as did Robert Boyle in 1671.
This bubbly discovery only gained attention in 1766, when English physicist Henry Cavendish collected the gas produced by the chemical reaction and showed that when ignited, this gas produced water. The name hydrogen derives from this fact; ‘hydro’ means water and ‘gen’ relates to formation or preceding. The name was given by Antoine Lavoisier.
Properties and applications of hydrogen
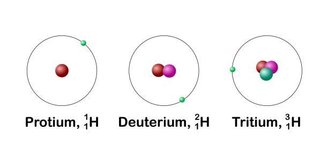
The properties of hydrogen have been studied over the years. For example, it was discovered Hydrogen has three isotopes: protium, deuterium, and tritium.The first is the usual representation of the element containing only one proton in its nucleus.
Deuterium, on the other hand, has one proton and one neutron in its nucleus, and finally, tritium, the radioactive form of hydrogen, has two neutrons and one proton. This molecular difference leads to differences in the mass of the element and how this isotope reacts when in contact with other molecules.
When isolated, hydrogen appears as a colorless, odorless, flammable gas. It represents a good combustion source with relative combustion efficiency. Although wells containing pure hydrogen have been found, it is most commonly obtained from refining processes..
There is even a color classification that reflects how sustainable the source is from which the hydrogen is extracted. For example, white hydrogen is gas extracted from a natural source, while gray hydrogen is gas extracted from fossil fuels.
However, in addition to being an alternative to fuels, hydrogen is also used in the form of ammonia (NH3) in the production of fertilizers. Therefore, the use of this element in industry is plural and serves not only as a fuel but also as a raw material..
Hydrogen: the fuel of the future?
Although it is an excellent alternative to fossil fuels, technology needs to develop in the storage and distribution sectors. Because it is a volatile and highly flammable gas, extra care must be taken when storing it..
Although the storage route is made through the energy produced in hydrogen power plants, batteries with sufficient autonomy to store and maintain high electrical potential loads for long periods of time have not yet been developed.
In addition to these aspects, the machines and engines used today will need to be subject to adaptations or modifications to guarantee the same performance and efficiency in industrial production.

Another limiting factor is the source of hydrogen extraction. When considering methods with a lower carbon footprint, the most sustainable sources would be water electrolysis and extraction of natural gas wells.
But all this demand requires continuous investment and tax incentives so that there is a real cultural change in the sector, Making the transition to sustainable energies such as hydrogen attractiveWho knew that an element with just one proton could be so revolutionary?
The first element of the periodic table, the subject of much of the world’s carbon footprint agenda around global sustainability and extinction, has been constantly at the forefront of discussions about the future of our planet, but it will still be several years before this journey is fully taken over by industry, transportation and other systems dependent on fossil fuels.
Liked the content? Tell us about it on social media. Until next time!
Source: Tec Mundo
I’m Blaine Morgan, an experienced journalist and writer with over 8 years of experience in the tech industry. My expertise lies in writing about technology news and trends, covering everything from cutting-edge gadgets to emerging software developments. I’ve written for several leading publications including Gadget Onus where I am an author.