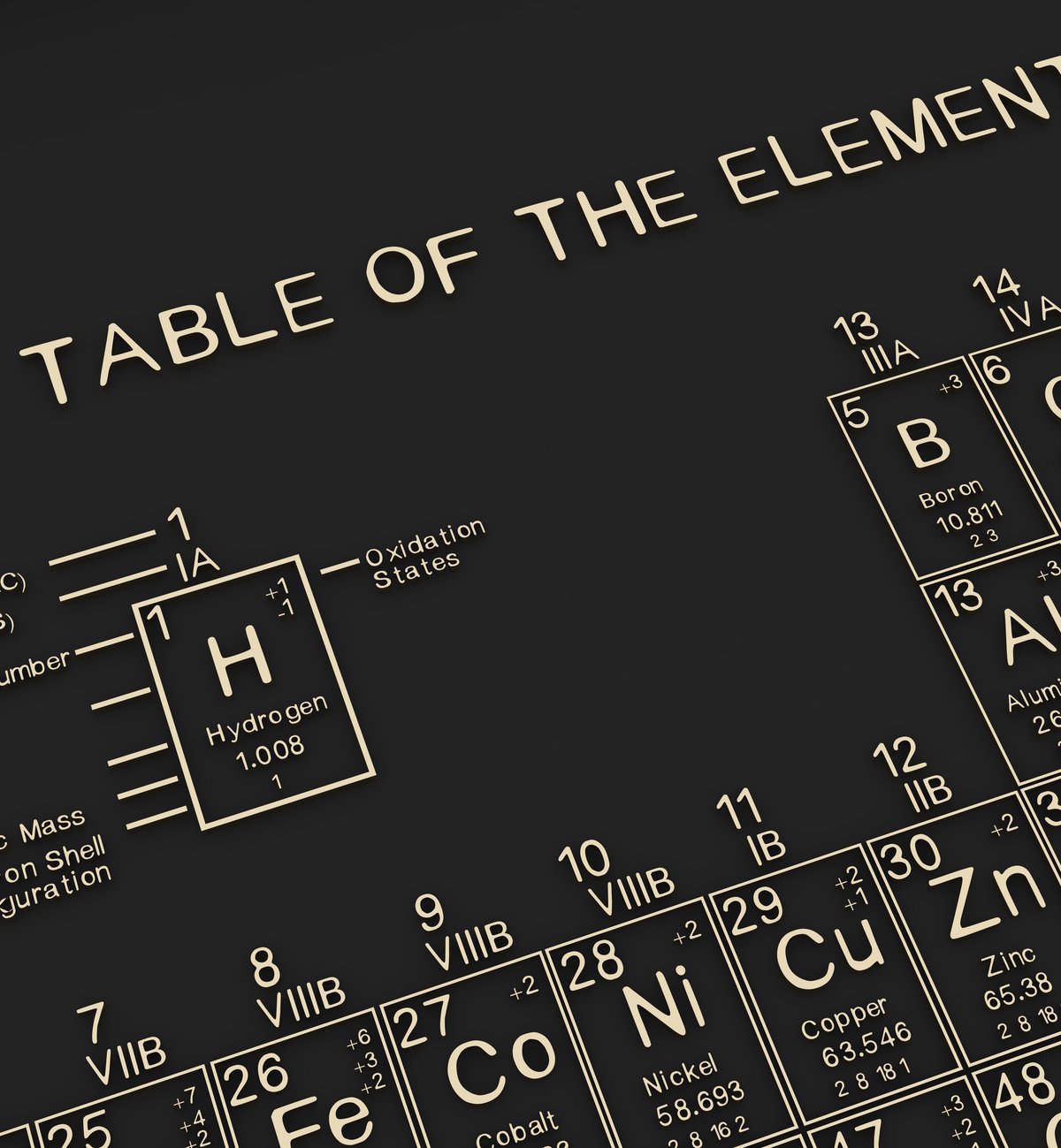
In the early days of elemental chemistry, it was still under the belief that atoms were the smallest part of matter. The all-important atomic number was no longer a concern. There have been some chemical element organizations discovered throughout history.
However, until the format we know today as the periodic table, proposed by Dmitri Mendeleev in 1869, elements were grouped according to their “essence,” such as whether they were metals, earths, or gases.
But under the scrutiny of scientists, chemists, experimenters and curious onlookers, a “magic” number emerged. A number that not only determines the location of each element on the periodic table but can also suggest new fundamental discoveries: the atomic number.
Anatomy of an atom
The first information about atoms was found in Greece in B.C. It dates back to the time of Democritus in the 5th century. Considered as the smallest piece of matter It was believed that these tiny pieces of the puzzle that make up the universe were indivisible, meaning that there was nothing smaller than atoms..
This idea persisted for centuries, surpassing Dalton’s Atomic Model (1803) and was proposed incompletely after Thomson’s famous “Plum Pudding” atomic model in 1897.
With the advancement of technology, it became possible to consider complete atomic formation, and today we know that the atom itself consists of even smaller pieces, clusters of leptons and quarks, known as fundamental particles.
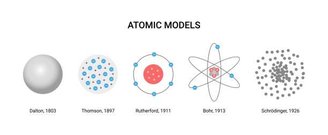
Of the particles previously considered fundamental: neutrons, protons, and electrons, that is, those that cannot be divided into even smaller parts, it is only electrons that are still considered indivisible. In a simple structure, all atoms consist of a set of protons, neutrons and electrons.
Currently the default model for studies is Schrödinger’s Atomic Model (1927), which includes concepts of quantum states of matter. However, the most commonly used atomic model in images is Niels Bohr’s model (1913), which is represented as the “planet model” thanks to its electron orbits.
One trick to rule them all
The concept of “atomic number” originates from the formation of the atomic nucleus. Contrary to popular belief, the number that accompanies the elements and determines their place in the periodic table is not random or in the order of discovery. Atomic number depends on the number of protons in each elementary nucleus..
But what is quite remarkable about the universe is that all atoms of a given element always have the same number of protons, regardless of whether there are differences in the number of neutrons or electrons available for that nucleus.

So, anywhere in the universe, an element with 23 protons in its nucleus will always be Vanadium, while an element with 98 protons will always be Californium. Besides, atomic number also helps researchers “discover” new elements.
Since we know that fundamental nature follows an order, the periodic table may one day have some additions to the evidence of only theorized elements where there are gaps today, such as the Unbiunium number 121.
In this way, researchers can not only make good predictions about the likelihood of new elements, but also hypothesize interactions that already cataloged elements might have with other elements.
The number of electrons and neutrons determines other features of the stabilization of the atomic nucleus. So we can say that The atomic number is the greatest indicator of the location, classification and degree of chemical and physical interaction of the elements..
New elements and new atomic numbers?
Although the table seems quite full with 118 elements, there is no limit to the inclusion of new elements. However, the heavier the elements, the less stable they are. Some of the last elements of the periodic table are so unstable that they survive for only milliseconds; This is a factor that hinders the ability to approve new elements.

But with the development of new technologies and ways to obtain elements, it is possible that new atomic numbers and related elements will soon become part of the periodic table.
But did you know that elements are organized according to the number of protons in the nucleus? Tell us about social media. Until later!
Source: Tec Mundo
I’m Blaine Morgan, an experienced journalist and writer with over 8 years of experience in the tech industry. My expertise lies in writing about technology news and trends, covering everything from cutting-edge gadgets to emerging software developments. I’ve written for several leading publications including Gadget Onus where I am an author.