Mendeleev’s periodic painting, which is an extremely powerful and indispensable tool for chemical operation, is unacceptable since 1869 to organize neutral chemicals based on chemical and spectroscopic properties. However, it cannot correctly define the electronic behavior of many loaded ions, that is, the atoms that lose many electrons.
In these atoms formed under extreme conditions such as high -energy physics laboratories or under -star ones, the interactions between the nucleus and the remaining electrons vary radically. With this, electronic features of these ions (energy levels, orbitals and light response) Do not comply with the logic of the traditional periodic table.
Now, a team of researchers at the Max Planck Nuclear Physics Institute in Heidelberg, Germany proposes an alternative element period, but focused on high -loaded ions. Chunhai Lyu, the first author of Chunhai Lyu, says, “We wanted to look for high -loaded ions to make it much more determined and much more accurate for atomic clocks,” Chunhai Lyu says. Reporter.
How does the classic periodic table work?
156 years ago, at a meeting of the Russian Chemical Association, during a meeting in São Petersburg, the periodic picture organized by Dmitri Mendeleev organizes 118 chemical elements known according to their characteristics. Scientists define existing gaps and discover new elements to fill them. This coordination facilitates the understanding of basic chemical properties.
In general, although it has done its functions for more than one and a half centuries for chemicals, it does not meet the demands of particles used in advanced technologies such as X -ray lasers, tumor therapies, plasma studies, physics theory tests and optical watches.
Since Rutherford and Bohr reinforce the concept of neutral atom, we know that the number of protons (positive load) is equal to electrons (negative load). However, atoms can lose or gain electrons by turning into loaded ions. When this loss of electron is large, the atom becomes a highly loaded ion, an important structure in special physical applications.
How is the “new” period organized?

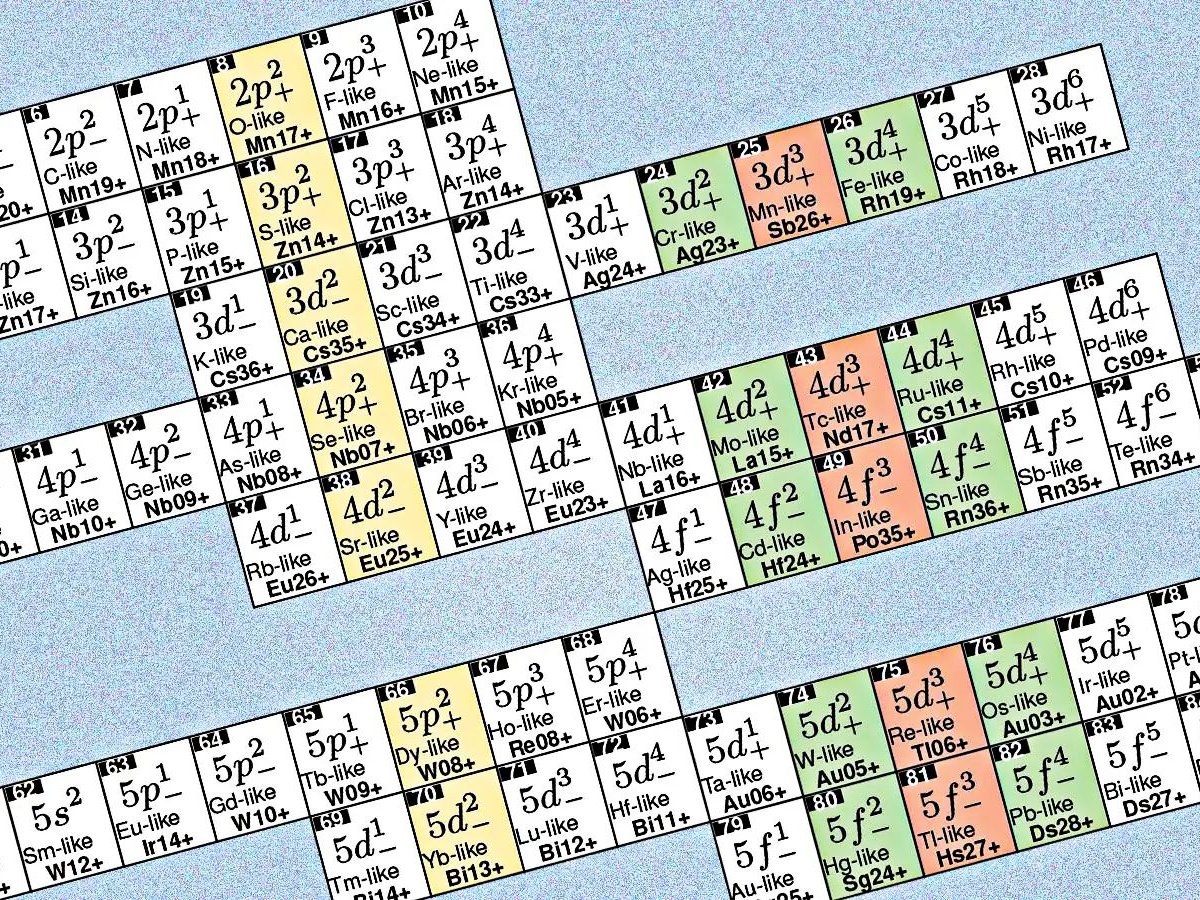
The first big difference between the classic periodic table and the “new” table is to recommend for high -loaded ions (English HCIs). This means that while Mendeleev’s table is ordered according to the number of protons in the atom of each element, Lyu and his colleagues are now grouping the ions of their ions according to their electronic structures and spectroscopic properties.
The new periodic picture does not organize neutral elements, but high -loaded atomsElectronic properties are different from the features foreseen by the classical table. This is due to its ability to achieve the same electronic configuration as another element when an atomic electrons lose electrons. Thus, each cell in the new table can group the ions of different elements that share these similar electronic structures, explains the LYU.
What the authors actually seek is rare and unusual transitions, absolutely not impossible, but quite possible and slow. They are very stable, known as “forbidden transitions ,, which makes them ideal for the optical atomic clock project. The reason for this is that in these cases, the atom can remain long for a long time before spreading or absorbing a photon.
New Work remains in the preliminary warehouse ArxivAnd it has not yet been revised by couples.
What did you think you had to deal with another periodic table? Comment on your social networks and enjoy sharing this article. Also, learn how the Ultra -Pre -Care measuring device combines atomic watches and quantum computers.
Source: Tec Mundo
I’m Blaine Morgan, an experienced journalist and writer with over 8 years of experience in the tech industry. My expertise lies in writing about technology news and trends, covering everything from cutting-edge gadgets to emerging software developments. I’ve written for several leading publications including Gadget Onus where I am an author.