A new paper published in the scientific journal Energy & Fuels reports that a team of scientists from the Institute of Energy and Environmental Chemistry (IQUEMA) at the University of Córdoba (UCO) in Spain, He managed to create the first battery that used ‘blood’ to produce energy. In fact, the developed prototype uses hemoglobin as a facilitator of electrochemical reactions and can therefore produce electrical energy.
To carry out the experiment, the researchers used zinc-air batteries that work through a chemical oxygen reduction reaction. Well, When air enters the battery, oxygen reduces the water at the positive end and releases the electrodes, which oxidizes the zinc at the negative end. — could be a much more sustainable alternative to existing lithium batteries.
It is precisely at this stage that ‘blood’ does its duty; A good catalyst with specific properties, such as hemoglobin, is required to drive the oxygen reduction reaction. By adding the protein found in red blood cells, they were able to create a battery prototype that continued to work for 20 to 30 days.
“To be a good catalyst in the oxygen reduction reaction, the catalyst must have two properties: It must absorb oxygen molecules quickly and form water molecules relatively easily. And hemoglobin met these requirements,” said study lead author and UCO researcher Manuel Cano Luna. in an official statement released to the press.
Hemoglobin battery
As mentioned earlier, hemoglobin facilitates the electrochemical reaction of the battery, and fortunately scientists only needed to use 0.165 milligrams of blood protein to sustain the study for up to 30 days. In addition to being a more sustainable option, Researchers explain that this can also be used in devices integrated into the human body, such as pacemakers.
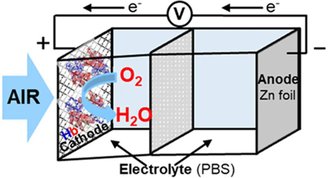
In any case, it is important to emphasize that this is still a prototype and proteins of animal origin will probably be used; Hemoglobin is present in almost all mammals. The next step for scientists will be to discover another protein that would make it possible to simply recharge the battery.
“The study, published in the journal Energy and Fuels, opens the door to new functional alternatives for batteries in a context where more and more mobile devices are expected and there is a growing reliance on renewable energy. There are devices that store energy in the form of chemical energy. Most importantly, today’s most common lithium-ion batteries are lithium It faces problems such as scarcity and its impact on the environment as hazardous waste,” he explains.
Did you like the content? Therefore, always stay up to date with more scientific curiosities at TecMundo. If you wish, take the opportunity to learn about all blood groups and the basic differences between them.
Source: Tec Mundo
I’m Blaine Morgan, an experienced journalist and writer with over 8 years of experience in the tech industry. My expertise lies in writing about technology news and trends, covering everything from cutting-edge gadgets to emerging software developments. I’ve written for several leading publications including Gadget Onus where I am an author.