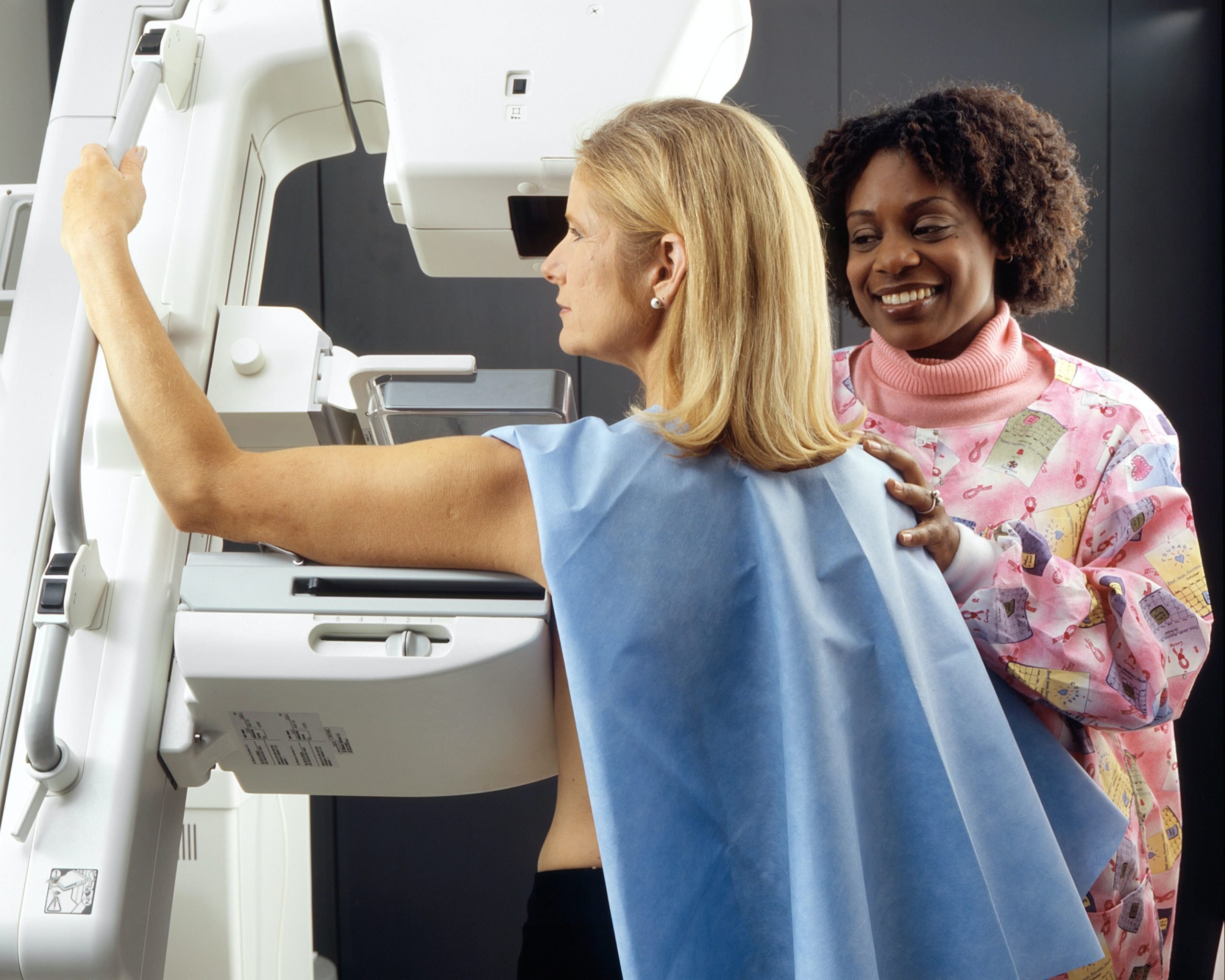
Reducing fossil fuel consumption involves, in particular, developing more Electric cars. Add to this the fact that more and more electronic devices are being developed that are designed to work without cables, and we can understand why. lithium batteries is becoming more and more relevant. Our daily life depends on them to a certain extent.
There are many types of batteries, but lithium batteries are the most widely used because of their efficiency and wide charging capacity. Unfortunately, this is not all the benefits, as lithium is a metal that is available in very few places in the world, and its production is associated with deforestation and destruction of ecosystems. If we want to reduce our consumption of fossil fuels, we must look for alternatives that do not harm the planet in other ways.
For this reason, many scientists devote their research to the development alternatives to lithium batteries. This is exactly the case with a group of scientists from the University of Cordoba, whose results were recently published. They managed to obtain a battery based on much cleaner and more accessible elements, such as sodium and sulfur. Besides being more environmentally friendly, how does this battery work?
Let’s start from the beginning: how lithium batteries work
Before we get into sodium and sulfur, it’s important to know how lithium batteries work. Basically, a battery, like a cell, works by forming electric current between two electrodes. The negative pole is known as the anode and the positive pole is known as the cathode. The flow of electrons passes through electrolytewhich acts as a bridge. Usually the cathode and anode are made up of a metal that loses electrons and another metal that accepts them. But there’s a problem: there comes a point when they run out. Who’s ever run out of batteries?
On the other hand, lithium batteries are rechargeable, which is a big advantage. But they are also very efficient. They are based on the fact that lithium is the element in the periodic table that gives up electrons most easily. When you lose them, rustsso that it forms positively charged lithium ion. It can then restore the electron and return to its stable form. And this is the main advantage of lithium batteries.
Usually they have a cathode cobalt oxide and the anode can have several options, but most often it will be a material similar to graphite, called coke. Lithium can be found in both electrodes along with these substances. However, the reaction begins at the anode. When a small current discharge occurs, the lithium atom in the anode becomes excited and loses an electron, forming a lithium ion. The lithium ions pass through the electrolyte, and the lost electrons form a chain on the outside of the battery. This flow of electrons provides the electricity needed to run cars or electronic devices.
What happens when the battery is dead because there is no more lithium to excite? No problem. The process can be created quite the opposite.
When we connect a lithium battery to an electric current, the electrons it supplies enter the external circuit and reach the cathode, where all the lithium ions are. They restore the lost electron and can return to the cathode to begin a new cycle.
Not all the benefits, but there are alternatives
We have already seen that lithium production is bad for the environment. But that is not the only disadvantage of lithium batteries. They also contain other substances that can be very toxic if thrown away.
For this reason, scientists from the University of Cordoba looked for an alternative based on sulfur and sodium. Its operation is simple. On the cathode there is only sulfur, and on the anode sodium. Both are elements safe, clean and very easy to get. In principle, this was a problem, since the sodium atom is much larger than the lithium atom and this significantly complicated its movement to the cathode. However, this problem was solved by adding metal organic framework (MOF) iron-based. Structures of this type have many applications, as their porosity makes them excellent adsorbents. They can attract many elements to themselves, and in this case this is useful, as it facilitates the movement of sodium ions to the cathode and their storage for later use.

The first tests of this alternative to lithium batteries have shown very positive results. It is clear that they support 2000 charging cyclesIf a person charges their mobile phone every 3 days, that would mean 120 charges per year, meaning the battery would last 15 years. Much longer than most of the ones we use today.
So if this can be done on a large scale, we will have an efficient and clean option for mobile phones or electric cars of the future. It certainly looks good, although we will have to wait to see how it develops.
Source: Hiper Textual