In the wake of creative chaos, everything in the universe is searching for some balance. At the atomic level, this stability can be achieved by donating or sharing electrons, forming chemical bonds.
These fundamental interactions bring elements together and shape their properties. As a result, various substances in the liquid, solid or gaseous state that we know in the universe emerge.. But they are not the same.
Today we’ll remember why chemistry is your favorite subject at school, and if you’re taking the entrance exam, be aware of the differences!
Everyone wants to be noble!
The simple anatomy of an atom reveals a nucleus surrounded by an electrosphere. The nuclear content consists of protons and neutrons, while the electrosphere consists of electrons in different energy shells.
These layers can be more or less stable depending on the number of electrons they support or need to protect themselves. In general, elements respond to a “law” called the Octet Rule.Where the vast majority of elements need at least eight electrons in their final valence shell to remain stable.
But the periodic table always has its unruly children, such as Beryllium, Lithium, Aluminum, Phosphorus, Sulfur and Xenon, which are examples of elements that do not follow the rules. These elements can undergo expansion or contraction of the terminal valence shell and become stable with more or fewer electrons.
There is also Hydrogen and Helium, which respond to the Duet Rule where their shells support a maximum of two electrons. There are many exceptions to many rules.
Octet Theory was proposed by American chemist and physicist Gilbert Newton Lewis (1875-1946), who observed interactions between various chemical compounds. He is known for his contributions to the understanding of acids and bases..
He observed that elements reach stability after completing eight electrons in their valence shell, just like the natural configuration of the noble gases.. Group 18 of the Periodic Table is known for its stability and low reactivity with other elements..
So how can other elements with fewer electrons in the final valence shell achieve this level of stability? Through chemical bonds! Let’s play electronic speculation.
I’m calling you because the greatest love of my life is chemistry
There are some types of chemical bonds, but today we will mainly focus on two: Covalent Bonds and Ionic Bonds. They have different properties and connect different elements. So stay tuned.
Covalent Bond
This type of bond is formed mainly through the sharing of electrons between non-metallic elements. A simple example is when siblings share the same wardrobe.
In this connection type, there is no change in the electronic distribution of the elements.In addition, the atoms to be bonded have the same degree of electronegativity.
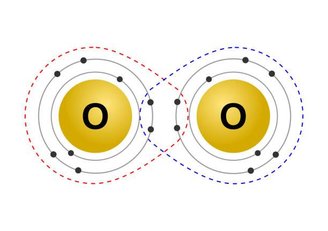
Covalent chemical bonds can be classified as simple when only one pair of electrons is shared, double when two pairs are shared, as in the picture above, and triple when three pairs of electrons are shared.
Compounds formed They are not always soluble in water due to covalent bonds and, with the exception of graphite, are generally poor conductors of electricity.. They can also be malleable and relatively resistant, at which point they gain strength from ionic bonds. And that’s what I’m talking about.
Ionic Bonds
The main feature of ionic bonds is the donation of electrons between metallic and non-metallic elements.. In this type of chemical bonding, there is a change in the electronic distribution of the elements because donating one or more electrically negative charges causes a change in the level of electronic distribution of the element and also a change in the polarity of the molecules. .
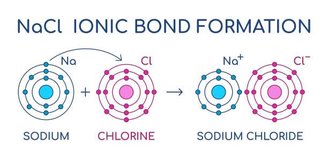
This means a bond between atoms with different degrees of electronegativity. This difference will continue after the donation, and the strength of the bond between these elements lies precisely in the attraction created by these differences.
Donors are not poor, they are stable. If we recall electronic distributions via the Linus Paulling Diagram, We know that electrons exist in different levels and that each level can be changed by donations or acquisitions between interactions of the elements.. If an element provides a particle, it is because it is present.
Other interesting factors about ionic bond compounds are their water solubility, high conductivity due to the movement of electrons, and high melting and boiling points due to the strong attraction between ions.
Ionic compounds form three-dimensional crystal lattices in which ions are held together by electrostatic forces. However, these structures can be a bit fragile. In addition to water solubility, external forces also they can “force” atoms to rearrange, leading to interactions between ions of equal polarity and repulsion of like poles. It was strong while it lasted.
Which chemical bond is the strongest?
I can already see the anticipation in your eyes to find out the outcome of today’s duel, but as always the answer is big: it depends! When we talk about force and chemical bonds we need to think about which aspects of force we are talking about..
Concerning the shape of the connection between one atom and another, ionic bonds increase due to reactions formed by electron donation, which creates a bonding force through the difference in polarity between molecules, among other factors.
Therefore, covalent chemical bonds also have their place; they are strong, resist dissolution, and are very stable. Additionally, compounds formed of this type are malleable and adaptable, and we have seen above that crystals with ionic bonds can be somewhat brittle.
The winner in this conflict will depend on the analyzed feature, but what can be said without a doubt? It is only through these wonderful, complex and diverse interactions that our world remains harmonious and functional..
If you were the jury, who would you give the award for the strongest chemical bond? Tell us about our social networks and when making your decision, you may want to include the carbon bond in the race to spark this conflict between chemical bonds!
I know there is chemistry between you and TecMundo, so stay connected by following our content. Until later!
Source: Tec Mundo
I’m Blaine Morgan, an experienced journalist and writer with over 8 years of experience in the tech industry. My expertise lies in writing about technology news and trends, covering everything from cutting-edge gadgets to emerging software developments. I’ve written for several leading publications including Gadget Onus where I am an author.